ATOMIC ABSORPTION INSTRUMNT SPECTROPHOTOMETER
HOME Email Webmaster
Related Links :
Graphite Furnace
Types of interferences
Interferences in AA analysis
Hg cold vapor and As hydride generation
1000 ppm AA standards
The Spectrophotometer
Atomic absorption is the determination of the presence and concentrations of metals in liquid samples. Metals include Fe, Cu, Al, Pb, Ca, Zn, Cd and many more. Typical concentrations range in the low mg/L (ppm) range.
In atomic absorption spectrometry, light of a specific wavelength is passed through the atomic vapor of an element of interest, and measurement is made of the decrease in the intensity of light as a result of absorption by atoms in the 'excited' state.
Metals will absorb ultraviolet light in their elemental form when they are excited by heat, either by flame or graphite furnace. Each metal has a characteristic wavelength that will be absorbed.
The sample of interest is aspirated and atomized into the flame. If that metal is present in the sample, its atoms will absorb some of the light, thus reducing its intensity. This decrease in intesity of the light is the process of atomic absorption. The instrument measures the change in intensity. A computer data system converts this change into an absorbance.
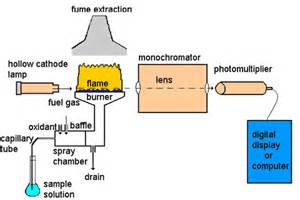
The atomic absorption spectrophotometer.
The following components make up the AA spectrometer :
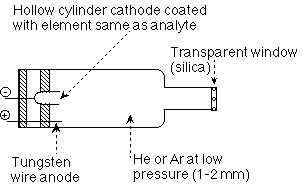
Hollow cathode lamp
The Hollow cathode Lamp is made with an element (metal) as the cathode encased in a bulb with a low pressure inert gas. It is imposed with a 10mA current such that when the metal is excited electrically, characteristic spectral line/lines are emitted and passed directionally through a glass UV/VIS window of the lamp.
This is the Source of the analytical light line for the element which is to be measured. It gives a constant and intense beam of the specific analytical line or wavelength of the element.
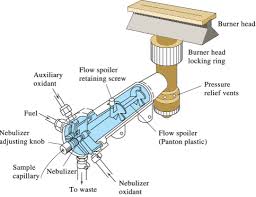
Nebulizer
The nebulizer sucks up liquid sample at a controlled rate, create a fine aerosol that mixes with fuel and oxidant for introduction into the flame.The nebulizer uses the combustion flames to atomize and introduce the sample into the light path. The high velocity of the combustion gases cause a negative pressure inside the nebulizer chamber and create a suction for an uptake tube by a process of aspiration. At the same time, the combustion gases rushing through the nubilizer draws liquid sample into the flow and is introduced into the flame as very fine droplets. Larger droplets are stopped by by baffles or spoilers and flow to waste. Excess aspiration liquid is removed by gravity and the waste is collected through an exit tube into a glass container. This waste is still highly acidic and care should be taken in its handling and disposal.
Flame
Temperature of some flames 0C Fuel Oxidant Temp0C Natural gas Air 1700-1900 H2 Air 2000-2100 C2H2 Air 2100-2300 C2H2 N2O 2600-2900 C2H2 O2 3050-3150
An oxidant and a fuel gas are mixed together and lit to create the flame for FAAS. The Flame is the Atomizer in which the sample undergoes desolvation and vaporization at high temperature. It destroys any analyte ions, break up complexes and creates the atoms of the element of interest, eg. Feo, Cuo, Zno, etc.
Two gas combinations are used. One is air/acetylene where the air is the oxidant and acetylene is the fuel. (23000C max.)
The other is nitrous oxide/aceylene in which the nitrous oxide is oxidant and acetylene is the fuel. (29000C max.)
Alkali metals, alkaline earths and some transition metals can be analysed with air/acetylene flames.
Refractory elements that form stable oxides and require hotter flames should be determined using the nitrous oxide/acetylene flame.
Acetylene gas cylinders may contain dissolved acetone and in-line filters must be installed.
A fuel-lean flame is an oxidizing flame of air-acetylele mixtures and is a blue, hot flame.
A fuel-rich flame is a reducing flame of air-acetylene mixtures and is a yellow, cool flame.
Burner Head
Burner heads must not be made with a composition of any of the metals determined by AA. It is normally made of solid Titanium which is corrosian resistant to strong acids and gases. Some burner heads used are:
All burners have means to make adjustments in height, foward-backward movement and angular rotation, in order to obtain optimum sensitivity under a give set of conditions.
- 1. The 10 cm single slot burner for air-acetylene flames. Its long burner path length provide best sensitivity.
- 2. The 5 cm single wide slot burner used for reduced sensitivity. This burner head can be used only for air-acetylene operation.
- 3. The 5 cm single slot burner used for nitrous oxide-acetylene flames. However, on some spectrometers, this burner can also be used with air-acetylene or air-hydrogen.
- 4. The 3 parallel slot burner used for those elements very difficult to atomize. The central flame is shielded by the outer flames and minimise cooling by entrained air.
For some elements that form refractory oxides (molecules hard to break down in the flame) nitrous oxide (N2O) needs to be used instead of air (78% N2 + 21% O2) for the oxidant. In that case, a slightly different burner head with a shorter burner slot length is used.
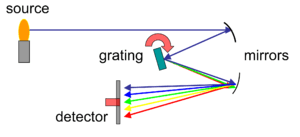
Monochromator
The main purpose of the monochromator is to isolate a single atomic resonance line (wavelength) from the lines emitted by the Hollow cathode lamp and transmit it to the detector. It not only selects the specific analytical line, but excludes all other interfering lines in that region.
Polychromatic light from the light source enters the monochromater and a dispersive device selects the wavelength to be measured and sends it through the exit slit to the detector. The dispersive device is a defraction grating which is a glass block machine-grooved in the order of 3000 grooves per millimeter and coated with highly reflected aluminium. Light striking these grooves are reflected and dispersed according to wavelength. By means of a selector knob on the monochromator a single analytical wavelength of choice can be selected and focused through the exit slit via the second mirror.
Photomultiplier tube (PMT)
This is the detector. The PMT determines the intensity of photons of the analytical line exiting the monochromator. Before an analyte is aspirated, a signal is generated by the PMT as a measurement of the light flowing from the HCL. When the sample is aspirated in the flame, some of this light is absorbed by "excited" atoms now present in the flame. This causes a decrease in PMT signal which is proportional to the amount of analyte. This decrease in light intensity by the atoms is referred to as atomic absorption. The signal from the PMT is converted to digital format by a transducer for read-out.
The PMT is the most commonly used detector for atomic absorption spectroscopy. However, solid state detectors are now replacing conventional vacuum-type photomultipliers. High tech electronics amplify, filter, and process the electrical signal, using a series of chips and microprocessors, transmitting the result to an internal or external computer which manage all data-handling and display.
Samples
The samples and standards are often prepared in duplicate with acid to match the analyte's chemical matrix as closely as possible. Acid contents of 1% to 10% are common. High acid concentrations help keep all dissolved ions in solution. The blank is a solution representative of the matrix of samples and standards but without the analyte.
Autosampler
With the autosampling accessory, tedious and time-consuming operations can be avoided. The autosampler is a computer-controlled, multi-purpose system. It automates standard and sample introduction for instrument calibration and analysis, and is a fully automated analytical workstation.
Wastes
Liquid sample not flowing into the flame collects on the bottom of the nebulizer chamber and flows by gravity through a waste tube to a glass waste container (highly acidic).
Shutdown
Before shutdown, aspirate deionized water through the system to flush out salts and acid. Then close off fuel first, then oxidant, and bleed gas supply cylinders. Most modern instruments control the ignition and shutdown procedures automatically.
Maintenance
The Atomic Absorption Spectrophotometer is designed to give accurate and precise results. The instrument set up and operation parameters should be observed as outlined in the Instrument Instruction Manual. However, occasional problems may arise that frustrate the less experienced operator. Most of these problems arise in the area of sample introduction and improper maintenance. Attention must be focused on lamps and burner, combustion gases, nebulizer tubing and drain assembly. A corrective diagnostic and maintenance program should be etablished in order to solve the problems associated with the instrument and the analytical method.
delloyd.50megs.comTroubleshoot the Atomic Absorption Spectrophotometer