Analysis and column problems in Gas Chromatography
This is a special page on gas chromatography, with some important practical and theoretical considerations: info on theory, stationary phases, capillary columns, analytical performance and troubleshooting.
GAS CHROMATOGRAPHY
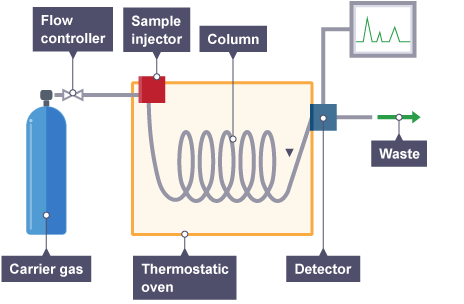
Analysis by gas chromatography is carried out by an instrument called the Gas Chromatograph. A compressed inert gas (carrier gas/mobile phase) is passed through a liquid sample into a heated coiled metal or glass tube coated with a stationary phase where each component is sequentially separated and sent to a detector to generate a chromatogram.
.
The Principles of Gas Chromatography
Chromatography is the separation of a mixture of compounds (solutes) into separate components. By separating the sample into individual components, it is easier to identify (qualitate) and measure the amount (quantitate) of the various sample components. There are numerous chromatographic techniques and corresponding instruments.Gas chromatography (GC) is one of these techniques. It is estimated that 10-20% of the known compounds can be analyzed by GC. To be suitable for GC analysis, a compound must have sufficient volatility and thermal stability. If all or some of a compound or molecules are in the gas or vapor phase at 400-450°C, and they do not decompose at these temperatures, the compound can probably be analyzed by GC.
One or more high purity gases are supplied to the GC. One of the gases (called the carrier gas) flows into the injector, through the column and then into the detector. A sample is introduced into the injector usually with a syringe or an exterior sampling device. The injector is usually heated to 150-250°C which causes the volatile sample solutes to vaporize. The vaporized solutes are transported into the column by the carrier gas. The column is maintained in a temperature controlled oven.
The solutes travel through the column at a rate primarily determined by their physical properties, and the temperature and composition of the column. The various solutes travel through the column at different rates. The fastest moving solute exits (elutes) the column first then is followed by the remaining solutes in corresponding order. As each solute elutes from the column, it enters the heated detector. An electronic signal is generated upon interaction of the solute with the detector. The size of the signal is recorded by a data system and is plotted against elapsed time to produce a chromatogram.
The ideal chromatogram has closely spaced peaks with no overlap of the peaks. Any peaks that overlap are called coeluting. The time and size of a peak are important in that they are used to identify and measure the amount of the compound in the sample. The size of the resulting peak corresponds to the amount of the compound in the sample. A larger peak is obtained as the concentration of the corresponding compound increases. If the column and all of operating conditions are kept the same, a given compound always travels through the column at the same rate. Thus, a compound can be identified by the time required for it to travel through the column (called the retention time).
The identity of a compound cannot be determined solely by its retention time. A known amount of an authentic, pure sample of the compound has to be analyzed and its retention time and peak size determined. This value can be compared to the results from an unknown sample to determine whether the target compound is present (by comparing retention times) and its amount (by comparing peak sizes).
If any of the peaks overlap, accurate measurement of these peaks is not possible. If two peaks have the same retention time, accurate identification is not possible. Thus, it is desirable to have no peak overlap or co-elution
RETENTION TIME (tR)
Retention time (tR) is the time it takes a solute to travel through the column. The retention time is assigned to the corresponding solute peak. The retention time is a measure of the amount of time a solute spends in a column. It is the sum of the time spent in the stationary phase and the mobile phase.COLUMN BLEED:
Column bleed is the background generated by all columns. It is the continuous elution of the compounds produced from normal degradation of the stationary phase. Column bleed increases at higher temperatures.COLUMN TEMPERATURE LIMITS:
Columns have lower and upper temperature limits. If a column is used below its lower temperature limit, rounded and wide peaks are obtained (i.e., loss of efficiency).
No column damage has occurred; however, the column does not function properly.
Using the column at or above its lower limit maintains good peak shapes.
Upper temperature limits are often stated as two numbers. The lower one is the isothermal temperature limit. The column can be used indefinitely at this temperature and reasonable column bleed and lifetime are realized.
The upper number is the temperature program limit. A column can be maintained at this temperature for 10-15 minutes without severely shortening column lifetime or experiencing excessively high column bleed.
Exposing the column to higher temperatures or for longer time periods results in higher column bleed and shorter column lifetimes. Exceeding the upper temperature limits may damage the stationary phase and the inertness of the fused silica tubing.
COLUMN CAPACITY:
Column capacity is the maximum amount of a solute that can be introduced into a column before significant peak distortion occurs.
Overloaded peaks are asymmetric with a leading edge. Overloaded peaks are often described as "shark fin" shaped. Tailing peaks are obtained if a PLOT column is overloaded. No damage occurs if a column is overloaded.
Types of gc Capillary columns
Capillary columna are known as Open Tubular columns. A thin film (0.1-10.0 micro meter) of thermally stable polymer is coated onto the wall of small diameter tubing (0.05-0.53mm id).Most columns are drawn from glass or silicate glass such as alkali-borosilicate glass, borosilicate glass, or alumina silicate glass. Other materials such as stainless steel, aluminium, copper, and even plastics have been used. However, each has its own relative merits according to the application.
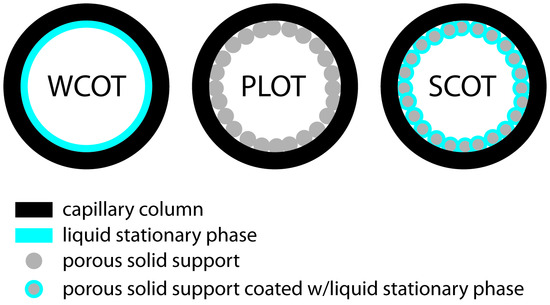
PLOT Porous Layer Open Tubular.
The stationary phase is the adsorbent, packing, or porous polymer. Only the support (packing) is added to the inner walls of the column.
PLOT columns are very retentive. They are used to obtain separations that are impossible with conventional stationary phases. Also, many separations requiring subambient temperatures with polysiloxanes or polyethylene glycols can be easily accomplished above ambient temperatures with PLOT columns.
Hydrocarbon and sulfur gases, noble and permanent gases, and low boiling point solvents are some of the more common compounds separated with PLOT columns.
Some PLOT columns may occasionally lose particles of the stationary phase. For this reason, using PLOT columns that may lose particles with detectors negatively affected by particulate matter is not recommended. Mass spectrometers are particularly susceptible to this problem due to the presence of a strong vacuum at the exit of the column.
SCOT Support Coated Open Tubular.
Both support (packing) and stationary phase are added to wall of column. Inner wall is lined with thin layer of support material such as diatomaceous earth which has been adsorbed with a stationary phase.Scot columns can hold a greater volume of stationary phase but still has a lower column efficiency than WCOT columns.
WCOT Wall Coated Open Tubular.
Only the stationary phase is added to the walls of this type of column.WCOT glass columns can be chemically etched by gaseous or concentrated acid treatment to provide a suitably rough surface for the stationary phase to bond more strongly.
FSWC Fused Silica Wall Coated Open Tubular column.
This is a special type of WCOT column, and is one of the most popular of the open tubular capillary columns. The walls of these columns are coated with a liquid stationary phase.The column is drawn from pure silica and is much thinner than glass columns. They have a diameter as small as 0.1mm and lengths up to 100m. The outside of the column is treated with a polyamide coating to protect it and allow it to be bent into coils to fit inside the oven of the gas chromatograph.
FSWC columns have great strength, flexibility, low reactivity, speed and efficiency.
COLUMN BREAKAGE:
Fused silica columns break wherever there is a weak point in the polyimide coating. The polyimide coating protects the fragile fused silica tubing. The continuous heating and cooling of the oven, vibrations caused by the oven fan and being wound on a circular cage all place stress on the tubing.Eventually breakage occurs at a weak point. Weak spots are created when the polyimide coating is scratched or abraded. This usually occurs when a sharp point or edge is dragged over the tubing. Column hangers and tags, metal edges in the GC oven, column cutters and miscellaneous items on the lab bench are just some of the common sources of sharp edges or points.
It is rare for a column to spontaneously break. Column manufacturing practices tend to expose any weak tubing and eliminate it from use in finished columns. Larger diameter columns are more prone to breakage. This means that greater care and prevention against breakage must be taken with 0.45-0.53 mm I.D. tubing than with 0.18-0.32 mm I.D. tubing.
A broken column is not always fatal. If a broken column was maintained at a high temperature either continuously or with multiple temperature program runs, damage to the column is very likely. The back half of the broken column has been exposed to oxygen at elevated temperatures which rapidly damages the stationary phase.The front half is fine since carrier gas flowed through this length of column. If a broken column has not been heated or only exposed to high temperatures or oxygen for a very short time, the back half has probably not suffered any significant damage.
A union can be installed to repair a broken column. Any suitable union will work to rejoin the column. No more than 2-3 unions should be installed on any one column. Problems with dead volume (peak tailing) may occur with multiple unions.
THERMAL DAMAGE:
Exceeding a column upper temperature limit results in accelerated degradation of the stationary phase and tubing surface. This results in the premature onset of excessive column bleed, peak tailing for active compounds and/or loss of efficiency (resolution).Fortunately, thermal damage is a slower process, thus prolonged times above the temperature limit are required before significant damage occurs. Thermal damage is greatly accelerated in the presence of oxygen. Overheating a column with a leak or high oxygen levels in the carrier gas results in rapid and permanent column damage.
Setting the maximum oven temperature at or a few degrees above the column temperature limit is the best method to prevent thermal damage. This prevents the accidental overheating of the column. If a column is thermally damaged, it may still be functional.
Remove the column from the detector. Heat the column for 8-16 hours at its isothermal temperature limit. Remove 10-15 cm from the detector end of the column. Reinstall the column and condition as usual. The column usually does not return to its original performance; however, it is often still functional. The life of the column will be reduced after thermal damage.
OXYGEN DAMAGE:
Oxygen is an enemy to most capillary GC columns. While no column damage occurs at or near ambient temperatures, severe damage occurs as the column temperature increases.In general, the temperature and oxygen concentration at which significant damages occurs is lower for polar stationary phases. It is constant exposure to oxygen that is the problem. Momentary exposure such as an injection of air or a very short duration septum nut removal is not a problem.
A leak in the carrier gas flow path (e.g., gas lines, fittings, injector) is the most common source of oxygen exposure. As the column is heated, very rapid degradation of the stationary phase occurs. This results in the premature onset of excessive column bleed, peak tailing for active compounds and/or loss of efficiency (resolution).
These are the same symptoms as for thermal damage. Unfortunately, by the time oxygen damage is discovered, significant column damage has already occurred. In less severe cases, the column may still be functional but at a reduced performance level. In more severe cases, the column is irreversibly damaged.
Maintaining an oxygen and leak free system is the best prevention against oxygen damage. Good GC system maintenance includes periodic leak checks of the gas lines and regulators, regular septa changes, using high quality carrier gases, installing and changing oxygen traps, and changing gas cylinders before they are completely empty.
CHEMICAL DAMAGE:
There are relatively few compounds that damage stationary phases. Introducing non-volatile compounds (high molecular weight or high boiling point) in a column often degrades performance, but damage to the stationary phase does not occur. These residues can often be removed and performance returned by solvent rinsing the columnInorganic or mineral bases and acids are the primary compounds to avoid introducing in a column. The acids include hydrochloric (HCl), sulfuric (H2SO4), nitric (HNO3), phosphoric (H3PO4) and chromic (CrO3). The bases include potassium hydroxide (KOH), sodium hydroxide (NaOH) and ammonium hydroxide (NH4OH).
Most of these acids and bases are not very volatile and accumulate at the front of the column. If allowed to remain, the acids or bases damage the stationary phase. This results in the premature onset of excessive column bleed, peak tailing for active compounds and/or loss of efficiency (resolution). The symptoms are very similar to thermal and oxygen damage.
Hydrochloric acid and ammonium hydroxide are the least harmful of the group. Both tend to follow any water that is present in the sample. If the water is not or only poorly retained by the column, the residence time of HCl and NH4OH in the column is short. This tends to eliminate or minimize any damage by these compounds. Thus, if HCl or NH4OH are present in a sample, using conditions or a column with no water retention will render these compounds relatively harmless to the column.
The only organic compounds that have been reported to damage stationary phases are perfluoroacids. Examples include trifluoroacetic, pentafluoropropanoic and heptafluorobutyric acid. They need to be present at high levels (e.g., 1% or higher). Most of the problems are experienced with splitless or Megabore direct injections where large volumes of the sample are deposited at the front of the column.
Since chemical damage is usually limited to the front of the column, trimming or cutting 1/2-1 meter from the front of the column often eliminates any chromatographic problems. In more severe cases, 5 or more meters may need to be removed. The use of a guard column or retention gap will minimize the amount of column damage; however, frequent trimming of the guard column may be necessary. The acid or base often damages the surface of the deactivated fused silica tubing which leads to peak shape problems for active compounds.COLUMN CONTAMINATION
Column contamination is one of the most common problems encountered in capillary GC. Unfortunately, it mimics a very wide variety of problems and is often misdiagnosed as another problem. A contaminated column is usually not damaged, but it may be rendered unusable.There are two basic types of contaminants: nonvolatile and semi-volatile. Nonvolatile contaminants or residues do not elute and accumulate in the column. The column becomes coated with these residues which interfere with the proper partitioning of solutes in and out of the stationary phase.
Also, the residues may interact with active solutes resulting in peak adsorption problems (evident as peak tailing or loss of peak size). Active solutes are those containing a hydroxyl (-OH) or amine (-NH) group, and some thiols (-SH) and aldehydes.
Semivolatile contaminants or residues accumulate in the column, but eventually elute. Hours to days may elapse before they completely leave the column. Like nonvolatile residues, they may cause peak shape and size problems and, in addition, are usually responsible for many baseline problems (instability, wander, drift, ghost peaks, etc.).
Contaminants originate from a number of sources with injected samples being the most common. Extracted samples are among the worse types. Biological fluids and tissues, soils, waste and ground water, and similar types of matrices contain high amounts of semivolatile and nonvolatile materials.Even with careful and thorough extraction procedures, small amounts of these materials are present in the injected sample. Several to hundreds of injections may be necessary before the accumulated residues cause problems. Injection techniques such as on-column, splitless and Megabore direct place a large amount of sample into the column, thus column contamination is more common wirh these injection techniques.
Occasionally contaminants originate from materials in gas lines and traps, ferrule and septa particles, or anything coming in contact with the sample (vials, solvents, syringes, pipettes, etc.). These types of contaminants are probably responsible when a contamination problem suddenly develops and similar samples in previous months or years did not cause any problems.
Minimizing the amount of semivolatiles and nonvolatile sample residues is the best method to reduce contamination problems. Unfortunately, the presence and identity of potential contaminants are often unknown. Rigorous and thorough sample cleanup is the best protection against contamination problems. The use of a guard column or retention gap often reduces the severity or delays the onset of column contamination induced problems.If a column becomes contaminated, it is best to solvent rinse the column to remove the contaminants.
Maintaining a contaminated column at high temperatures for long periods of time (often called baking out a column) is not recommended. Baking out a column may convert some of the contaminating residues into insoluble materials that cannot be solvent rinsed from the column. If this occurs, the column cannot be salvaged in most cases.
Sometimes the column can be cut in half and the back half may still be useable. Baking out a column should be limited to 1-2 hours at the isothermal temperature limit of the column.
Back to Top
Reference : J & W Catalogue "Gas chromatography" 1998.
Click this link for GC troubleshooting guide GC troubleshooting
Click this link for similar and comparison GC phases COMPLEMENTARY STATIONARY PHASES
Email About Main index Copyright DisclaimerBack to Top
Signature : Delloyd's Lab Tech resources, reagents and Instrumentation